Description
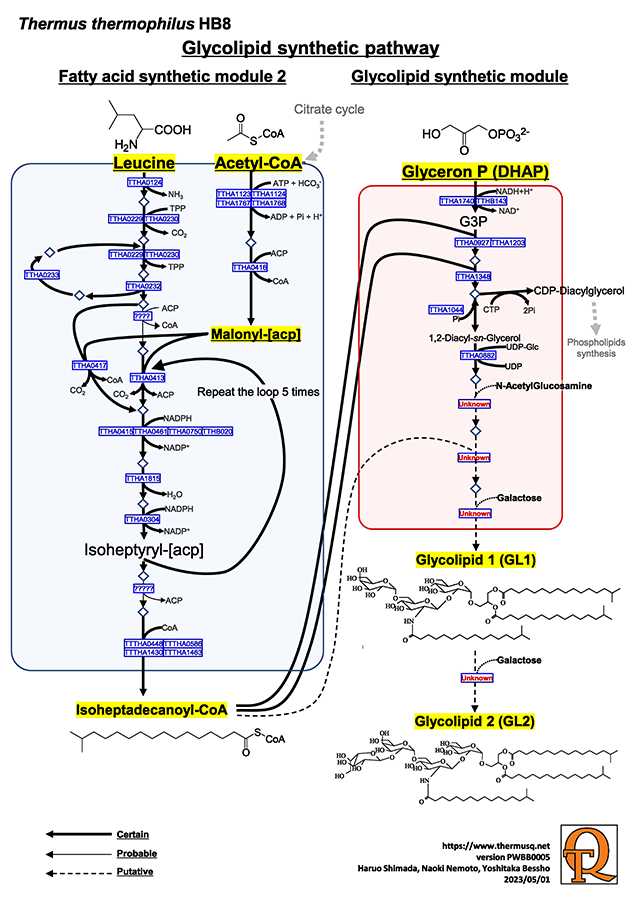
The lipid molecules in the cell membrane of Thermus thermophilus have numerous characteristics suitable for a high temperature environment compared with the standard phospholipids of mesophilic bacteria, such as the addition of iso or ante-iso branched fatty acids, the difference in the number of hydrophobic fatty acid side chains (three instead of two), a glycolipid as the major lipid and so on. In addition, a monosaccharide is attached to a T. thermophilus phospholipid and forms a phosphoglycolipid. The structures of the three main lipid molecules, namely two glycolipid molecules and one phosphoglycolipid molecule, have been determined. In particular, glycolipids (GL1 and GL2) are synthesized from two types of hydrocarbon chains.
The glycolipid biosynthesis pathway originates with leucine as a starting material, and isovaleryl-CoA is initially synthesized. Malonyl-CoA, which is synthesized from acetyl-CoA, binds to isovaleryl-CoA to form branchedchain-acetyl-[acp]. Then, it is converted to isoheptyl-[acp] and further combined with malonyl-CoA again, increasing the carbon chain length by C2 (forming a loop reaction). This loop is repeated 6 times, and a terminally branched fatty acid (iso-C17:0) is synthesized. If the loop is repeated 5 times, iso-C15 is synthesized. When isoleucine or valine are used as starting materials instead of leucine and loop is repeated 7 times, anteiso-C17:0 or iso-C16:0 are synthesized, respectively.
References
- Bagautdinov B, Ukita Y, Miyano M, Kunishima N, (2008) Structure of 3-oxoacyl-(acyl-carrier protein) synthase II from Thermus thermophilus HB8. Acta Crystallogr Sect F Struct Biol Cryst Commun, 64, 358–66.
- Nakai T, Nakagawa N, Maoka N, Masui R, Kuramitsu S, Kamiya N, (2004) Ligand-induced conformational changes and a reaction intermediate in branched-chain 2-oxo acid dehydrogenase (E1) from Thermus thermophilus HB8, as revealed by X-ray crystallography. J Mol Biol, 337, 1011–33.
- Otero JM, Noël AJ, Guardado-Calvo P, Llamas-Saiz AL, Wende W, Schierling B, Pingoud A, van Raaij MJ, (2012) High-resolution structures of Thermus thermophilus enoyl-acyl carrier protein reductase in the apo form, in complex with NAD+ and in complex with NAD+ and triclosan. Acta Crystallogr Sect F Struct Biol Cryst Commun, 68, 1139–48.
- Ogawa T, Suwanawat N, Toyotake Y, Watanabe B, Kawamoto J, Kurihara T, (2020) Lysophosphatidic acid acyltransferase from the thermophilic bacterium Thermus thermophilus HB8 displays substrate promiscuity. Biosci Biotechnol Biochem, 84, 1831–1838.
- Kawaguchi M, Shimada H, Bessho Y, Nemoto N, (2023) Profiling of lipids in Thermus thermophilus HB8 grown under various conditions. J Gen Appl Microbiol, 69, 79–90.
- Shimada H, Yamagishi A, (2011) Stability of heterochiral hybrid membrane made of bacterial sn-G3P lipids and archaeal sn-G1P lipids. Biochemistry, 50, 4114–20.
- Suda Y, Okazaki F, Hasegawa Y, Adachi S, Fukase K, Kokubo S, Kuramitsu S, Kusumoto S, (2012) Structural characterization of neutral and acidic glycolipids from Thermus thermophilus HB8. PLoS One, 7, e35067.