Description
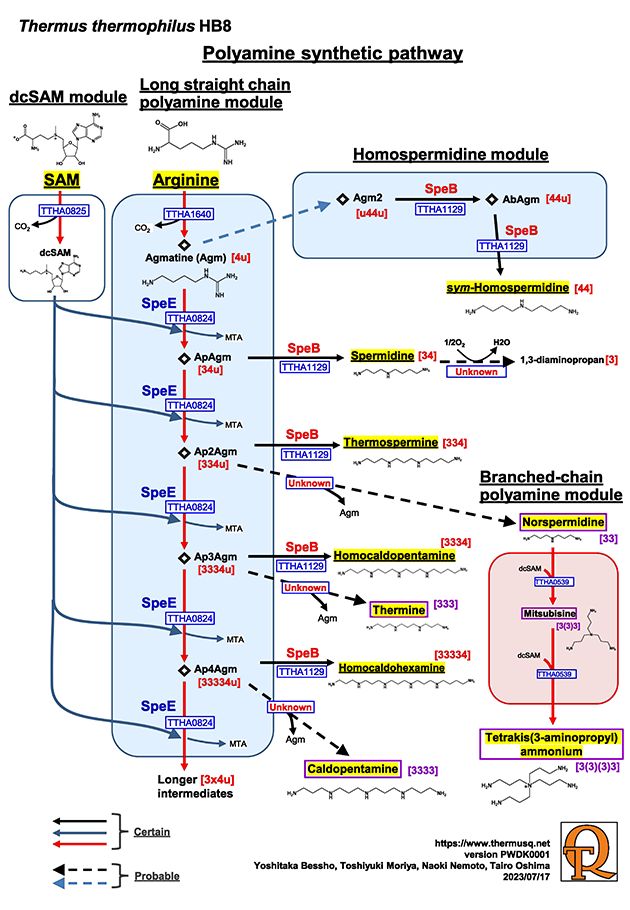
Thermus thermophilus produces a variety of unusual polyamines, including long-chain polyamines such as caldopentamine and sym-homospermidine, as well as branched polyamines like tetrakis(3-aminopropyl)ammonium. These unusual polyamines are thought to be essential for the thermostabilization of extreme thermophiles. The polyamine biosynthetic pathway in T. thermophilus differs from that of many other microorganisms, such as Escherichia coli. Arginine serves as the starting material and is converted into agmatine by the action of arginine decarboxylase, encoded by the speA gene. The agmatine is then aminopropylated in the presence of decarboxylated S-adenosylmethionine to produce aminopropyl agmatine. In T. thermophilus, spermidine, which is typically synthesized from putrescine in many other organisms, is produced by hydrolyzing this aminopropyl agmatine. Notably, T. thermophilus contains little to no putrescine. Long-chain polyamines are synthesized through the following two-step process. The first step involves the addition of an aminopropyl group to agmatine by an aminopropyltransferase encoded by the speE gene. Multiple aminopropyl groups can be added to produce longer-chain polyamines. The second step involves the removal of a urea moiety from the product of the first step. This reaction is catalyzed by an aminopropylagmatinase encoded by the speB gene.
References
- Hidese R, Fukuda W, Niitsu M, Fujiwara S, (2018) Identification of Branched-Chain Polyamines in Hyperthermophiles. Methods Mol Biol, 1694, 81–94.
- Oshima T, (2007) Unique polyamines produced by an extreme thermophile, Thermus thermophilus. Amino Acids, 33, 367–372.
- Ohnuma M, Terui Y, Tamakoshi M, Mitome H, Niitsu M, Samejima K, Kawashima E, Oshima T, (2005) N1-Aminopropylagmatine, a New Polyamine Produced as a Key Intermediate in Polyamine Biosynthesis of an Extreme Thermophile, Thermus thermophilus. J Biol Chem, 280, 30073–30082.
- Oshima T, Moriya T, Terui Y, (2011) Identification, Chemical Synthesis, and Biological Functions of Unusual Polyamines Produced by Extreme Thermophiles. , , 81–111.
- Oshima T, (2010) Enigmas of biosyntheses of unusual polyamines in an extreme thermophile, Thermus thermophilus. Plant Physiol Biochem, 48, 521–526.
- Kobayashi T, Sakamoto A, Hisano T, Kashiwagi K, Igarashi K, Takao K, Uemura T, Furuchi T, Sugita Y, Moriya T, et al., (2024) Caldomycin, a new guanidopolyamine produced by a novel agmatine homocoupling enzyme involved in homospermidine biosynthesis. Sci Rep, 14, 7566.